
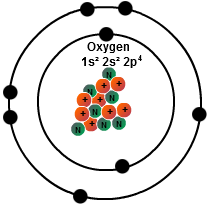
How Many Electrons Are in the Third Energy Level? If element x has 46 protons, how many electrons does it have? How many valence electrons does Magnesium (Mg) have? Beryllium has an electronic configuration of 1s2 2s2 in its natural state. How many of those electrons are unpaired? Atoms must have equal numbers of protons and electrons. The amount of protons and neutrons remains the same at 4. It is a noble gas, meaning it has the maximum number of valence electrons its outer shell can hold. Two electrons in the S-orbital and 7 in the D-orbital. The s sub-shell can hold no more than 2 electrons, the p sub-shell can hold 6, the d sub-shell can hold 10 and the f sub-shell can hold as many as 14. Answers: 2 Show answers Another question on Biology. Xenon, atomic number 54, group 18, noble gas, all noble gas has valence electrons of 8, and since it is in group 18, so 18 -10 = 8 is the number of valence ( this even applicable for anything in groups 10 to 18 for which you take the group s and subtract 10, that is the number of valence electrons. However, if the element includes a negative or positive ion, then the protons and electrons will not be the same. Then we have 2b level, which can hold up to 6 electrons. By Staff Writer Last Updated 4:47:16 AM ET. How many electrons does krypton have in its next-to-outer shell (n = 3)? The protons and electrons cancel each other out because protons produce a positive charge, electrons a negative charge, and they have the same amount. basically looking at the periodic table What is the lowest energy shell called? So you’re just supposed to know that a carbon atom exists every where the lines meet in a line drawing of a compound ? The electronic configuration of manganese is 3d5 4s2. 3.How many valence electrons does oxygen have? They have eight electrons in their valence shell, so noble gases are very unreactive. Which of the statements correctly describes the reactivity of noble gases, according to the octet rule? How many valence electrons are in a krypton cation with a 2 charge? 1s22s22p63s23p63d104s24p6 The valence shell of krypton is 4th, hence, there are 8 electrons in this valence shell. Find an answer to your question “The atomic number of krypton (Kr) is 36, and its mass number is 84.How many neutrons does it have? To do this, you need to write in the search box (for example, google) how many protons neutrons and electrons does lithium have and add to it an additional word: converter or calculator. How many core electrons does Cesium have? The atoms' valence electrons are shared between. Rows(Left to right) on the periodic table. The element's group number provides a clue about the number of valence electrons. Therefore, an element in a neutral state will have the same number of protons and electrons.

By Staff Writer Last Updated 12:36:52 AM ET. convert to the equivalent pressure in atmospheres,4.32*10^5 N/m^2. Which tends to increase genetic variaition in a population. Answer (1 of 4): 18 protons, 18 electrons, and 22 protons. they do not gain or lose electrons readily. how many unpaired electrons are in krypton? Many of you have this question of how many electrons are in the last cell of carbon or how many valence electrons does carbon have? among the following reactions find those that are redox reactions? Get unlimited access to 3.7 million step-by-step answers. Join Yahoo Answers and get 100 points today. Give the number of valence electrons for atoms of oxygen, sulfur, arsenic, p… 01:37. The group number tells you how many electrons are in … The electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6. You can view more similar questions or ask a new question. The element manganese has seven valence electrons. If you need to know how the electrons are arranged around an atom, take a look at the 'How do I read an electron configuration table?' Answered 3 hours ago. I know its 8 because it's a noble gas but how do you get the answer 8?Krypton has 36 electrons. Solved: How many valence shell electrons does an atom of indium have? :) … Similarly, what is the charge of mercury that has 80 electrons? How many valence electrons does #F# have? For example, boron (B) has an atomic number of 5, therefore it has 5 protons and 5 electrons. Krypton is atomic number 36, which means it has 36 protons in the nucleus and 36 electrons in orbit around the nucleus. 28.5 - Krypton (atomic number 36) has how many electrons.


 0 kommentar(er)
0 kommentar(er)
